Supporting energy security, sustainability and industrial growth
Transitioning to a low-carbon, resilient energy system requires rapid innovation. Scalable and cost-effective technology solutions will be needed for wind, energy storage, hydrogen and new nuclear.
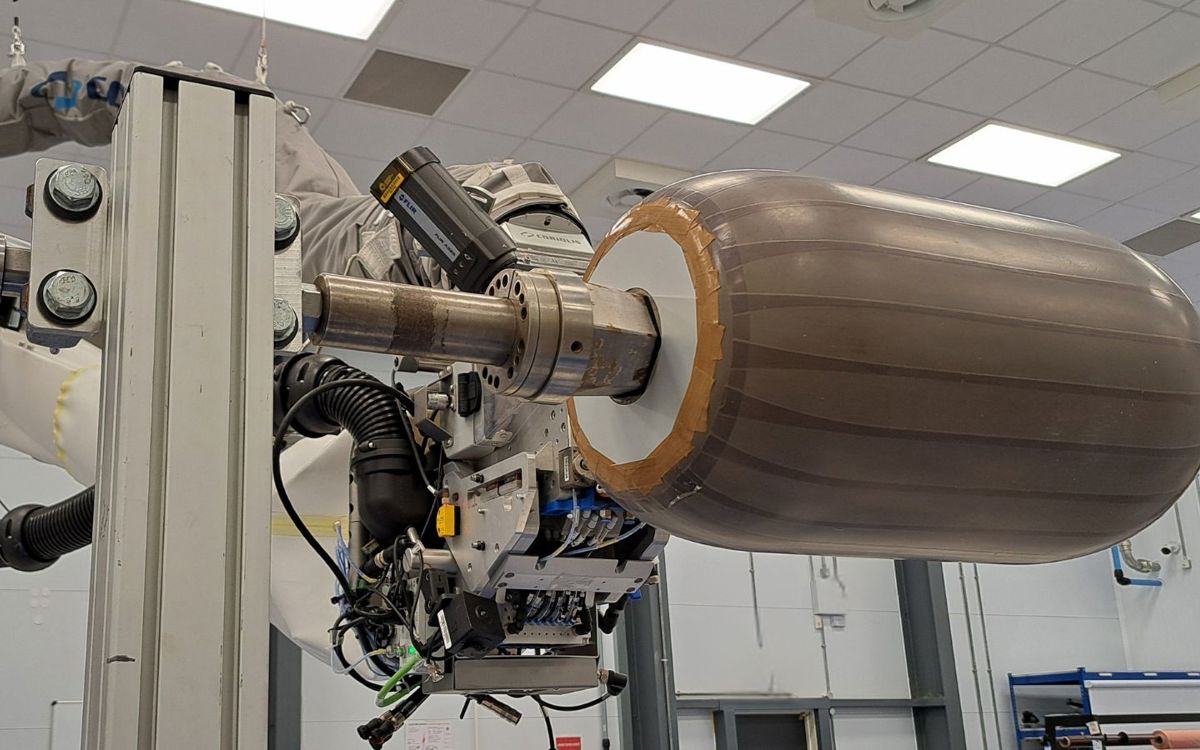
NCC is at the forefront of innovation across the energy sector. This includes wind energy generation technologies, hydrogen storage solutions, materials development for new nuclear and distribution technologies in oil and gas.
We are the UK’s centre of excellence for composite structures and a leader in the development and processing of novel materials. With experience across the product engineering life cycle – underpinned by unique digital capabilities and partnerships with global leaders from across the energy sector – we offer innovative solutions tailored for the energy transition.
Get In Touch
Connect with our Energy Specialists at NCC to find out more about our world-leading innovation capabilities and how we can support your business.
Energy focus areas across the technology and product life cycle
Major Programmes
We connect technology strategy to real-world delivery through our end-to-end engineering services